In a recent study published online by Neuron on January 8, 2025, a research team from Zhejiang University School of Medicine and Zhejiang Chinese Medical University, led by Professors HU Weiwei and CHEN Zhong, revealed new insights into Attention Deficit Hyperactivity Disorder (ADHD). The study, for the first time, reveals that the lack of histamine H2 receptors (H2R) on parvalbumin-positive (PV+ ) neurons leads to hyperactivity, impulsivity, and attention deficits in mice, facilitating understanding the underlying mechanisms of ADHD. This study also provides a new precise drug target for ADHD treatment.
ADHD is a common neuropsychiatric disorder that affects around 4% of the
global population, with core symptoms including inattention, hyperactivity, and impulsive behaviors. It is widely believed to be associated with dysfunction in the dopamine system. Currently available medications, such as methylphenidate, can be effective; however, they are associated with several side effects, including the potential for addiction.
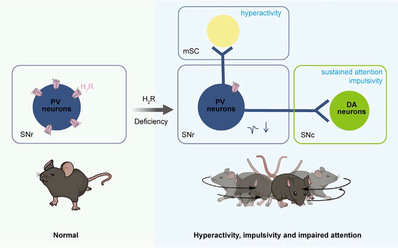
Thus, it is urgent to explore new mechanisms of ADHD and identify specific drug targets. The research team, which has been focusing on the role of histamine receptors in major brain diseases for a long time, selectively deleted the H2R from PV+ neurons by using Cre/loxp technology. The results demonstrated that these mice display hyperactivity, impulsivity, and attention deficits, while other functions, such as mood, learning, memory, and social behavior remain unaffected. Further analysis of both ADHD mouse models and human patients with ADHD revealed a significant decrease in H2R expression on PV+ neurons in the substantia nigra pars reticulata (SNr). Following specific knockdown of H2R expression in SNr PV+ neurons, researchers observed similar ADHD-like behaviors in mice.
Interestingly, administration of an H2R agonist to the SNr could alleviate these
behavioral abnormalities. The team subsequently employed in vivo single-cell recordings, ex vivo electrophysiology, optogenetic recordings, and chemogenetic manipulations to investigate the neural circuits that underlie these behaviors.
They discovered that the lack of H2R reduced the excitability of PV+ neurons, which in turn disinhibited downstream dopamine neurons in the substantia nigra pars compacta (SNc) and neurons in the medial layer of the superior colliculus (mSC), leading to the manifestation of ADHD-like behaviors.
Interestingly, the study revealed that the SNc-projecting and mSC-projecting
PV+ neurons play distinct roles in the regulation of ADHD-like behaviors.
These findings not only enhance our understanding of the pathophysiology
of ADHD, but also reveal a novel role for H2R in the brain. H2R in PV+ neurons
serves as a potential drug target for ADHD treatment.